我是参考这个 还有这个
The value of enthalpy of neutralisation depends on the strength of the reactant acids and bases in a particular reaction.
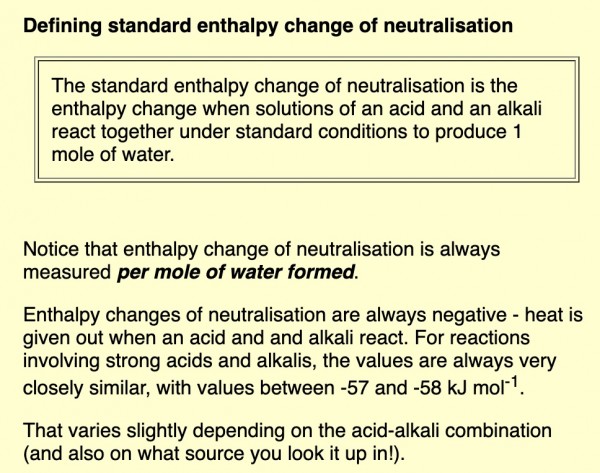
If the reactants are strong acids/strong bases, complete dissociation of molecules takes place and the enthalpy of neutralisation for strong acid + strong alkali are always very closely similar, with values between -57 and -58 kJ mol-1 (consider constant)
这句话的意思 是 无论你用哪一种 stong acid 或 stong alkali, 它释放出来的 heat of neutralisation 几乎都会是 -57 kJ mol-1
See this definition.
But in the case of a weak acid or weak base, some of the liberated heat is utilised to break the molecular bonds. Hence, the absolute value of enthalpy decreases with the strength of acids and bases.
如果不是 strong acid + strong alkali 的组合,它的 enthalpy of neutralisation 就会是个变数,不可能是 fixed value 的, 而且一定少过 -57 kJ mol-1
所以,你的图表中,只有第一行 是 fixed value (因为它是 stong acid + strong alkali), 其它的都是一个 example.
Conclusion
Enthalpy of neutralisation depends on the degree of dissociation of acids and bases in a reaction mixture, which in turn depends on their strength.
heat of neutralisation 完全取决于 acid/alkali 在溶液里的分解程度,而分解程度则是取决于 acid/alkali 本身的strength and concentration.
所以如果有两种 mixture, 两个都是属于 strong acid + weak alkali, 这两个的 heat of neutralisation 也未必会一样
你的图表只是一个 example, not necessary strong acid + weak alkali will produce more heat than a weak acid + strong alkali combination and vice versa
希望这个能帮助到你,也建议你去找老师讨论和确定一下。