Based on the equation, 1 mol of calcium carbonate is dissolved into 1 mol of carbon dioxide, so A is wrong.
Based on the equation, calcium carbonate is dissolved but not formed, so I think the question is wrong, if you change to calcium oxide then thats the exact value (see the calculation below)
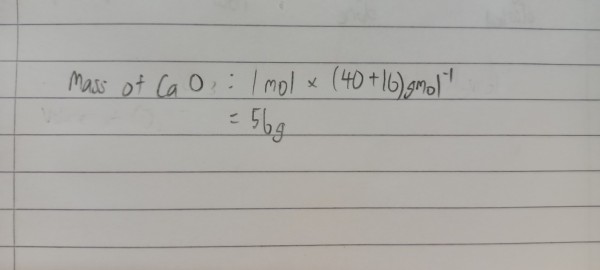
1 mol of carbon dioxide is formed so C is ridiculous
There's only 1 mol of carbon dioxide so there's only 24dm3 of it, D is wrong